
Grupo LIVERGEL – Liver cell models based on decellularized printable hydrogels for high-throughput drug screening.
A PROMETEO PROGRAM 2023 Project. Prometeo CIPROM 2022/43.
Funded by:

RESEARCH TEAM
Gloria Gallego Ferrer (IP) – UPV
José Luis Gómez Ribelles (IP) – UPV
M. Teresa Donato Martín – IISLaFe
José Antonio Gómez Tejedor – UPV
Manuel Salmerón Sánchez – UPV
Laia Tolosa Pardo – IISLaFe
Isabel Tort Ausina – UPV
Ana Vallés Lluch – UPV
GENERAL AIM AND OBJECTIVES
Drug-induced liver injury (DILI) is a patient-specific, multifactorial pathophysiological process that needs to be recapitulated in current in vitro models as best as possible, to improve safety assessment in the drug development process and diagnostics applications. Given the multifactorial mechanisms of DILI, which contribute to drug attrition in development and in the clinical practice, there is a need for new liver models that improve physiological relevance and more accurately predict and diagnose human drug-induced hepatotoxicity.
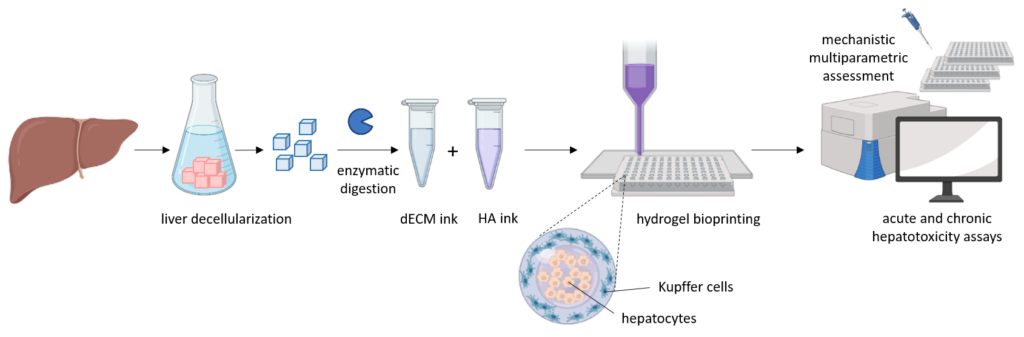
Our aim is to develop and validate a new biomimetic 3D cellular liver model from an integrated approach, based on printable hydrogels and different cell sources, and able to better recapitulate the hepatic functionality in vitro. Our proposed printable systems mimic the native liver microenvironment and will allow a high-throughput prediction of drug toxicity. We will develop physiologically relevant hydrogels from a combination of solubilized liver decellularized extracellular matrix and hyaluronic acid, which will provide printability by enzymatic crosslinking. Our material systems will be optimized to reproduce the native liver microenvironment with special emphasis in fine tuning mechanical properties, improving in vitro hepatocyte functions and maintaining liver-specific phenotype over longer periods. Hybrid bioinks will be developed for automatic bioprinting, looking for scalability, reproducibility and high-throughput toxicity studies. The protocols optimized with porcine tissue will be adapted to human tissue obtained from the biobank for a more compatible technology. We will test and develop our liver models using different hepatic cell sources, including induced pluripotent stem cells (iPSCs) from different human donors. Liver cells alone or in co-culture with other non-parenchymal cells will be encapsulated within the hydrogels and their functionality will be assessed by different omics technologies (trasncriptomics, metabonomics, etc.).
This is a breakthrough approach that will also be used to enhance the functional maturity of iPSCs derived hepatocyte-like cells (HLCs) and improve the performance of cell liver models. Finally, we will validate the potential of the platform to predict hepatotoxic events, by evaluating the effects induced by drug model compounds (hepatotoxic and nonhepatotoxic) after acute and chronic exposure, which is a more relevant scenario for therapeutics. This will allow us to design screening applications that consider the variability of human responses to drugs and also personalised diagnostics and prognosis of DILI in the clinical setting.
